Mostafa Ragab Abd El Wahab, Moaa Korany Seliem, Essam Abd El Rahman Mohamed, Ali Quarny seliem, Mohamed Gad Shahien
Abstract
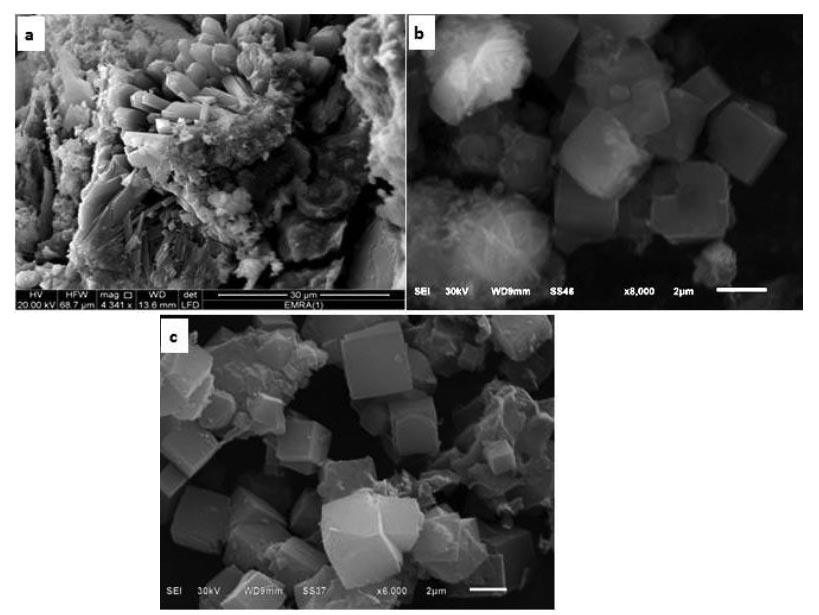
In this study, we prepared hydrated and dehydrated Na-zeolites under different conditions using sodium hydroxide (NaOH) and Egyptian kaolin. The synthetic zeolites were characterized by XRD, SEM, specific surface area and their adsorption behavior in the uptake of Cu2+ ions from aqueous solution were investigated, in order to compare the obtained results. The dehydrated Na-A zeolite showed well developed crystals and high surface area compared to hydrate Na-A zeolite. Batch adsorption studies showed that the maximum Cu2+ uptake percentage of dehydrated Na-A zeolite (94%) has been recorded after 5 min of contact time. On the other hand, the removal percentage of hydrated Na-A zeolite was 75% after 5 min reaching its maximum uptake percentage (97%) after 120 min. The mechanism of cooper ions uptake by dehydrated Na-A zeolite followed ion exchange and precipitation while ion exchange was the main mechanism of adsorption in the case of hydrated Na-A zeolite. Copper adsorption kinetics by the synthetic Na-A zeolites was well fitted by pseudo-second-order kinetic model with R2 greater than 0.99. Equilibrium sorption isotherms of Cu2+ ions by the synthetic zeolites were explained using the Langmuir and Freundlich isotherms models.
source: ijbio.com