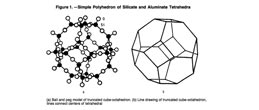
Ruder Boskovic Institute, Zagreb, Croatia
I. INTRODUCTION
A particular structural feature of zeolites relative to other aluminosilicate materials, and other crystalline materials in general, is the existence of channels and/or cavities linked by channels. One of the characteristics that distinguishes zeolites from other porous materials is their variety of pore sizes and shapes. The size and shape of channels/cavities in zeolites therefore define the structural parameters of a given type of zeolite (1). Properties of zeolites, such as ion exchange, intercrystalline pores that discriminate between molecules of different dimension, strong acidic sites, and active reservoirs for metal-catalyzed reactions, have earned them extensive industrial uses. Consequently, fundamental zeolite research has become an area of great interest (2). The remarkable applicability of zeolites ranges from uses in biochemistry, the agroindustry, detergents, soil improvements, the nuclear industry, energy storage, and the textile industry (3).
Zeolites are among the most important inorganic cation exchangers. The alumino- silicate structure is negatively charged and attracts cations that come to reside inside the pores and channels. Zeolites have large empty spaces, or cages, within their structures that can accommodate large cations, such as Na+, K+, Br+, and Ca2+, and even relatively large molecules and cationic groups, such as water, ammonia, carbonate ions, and nitrate ions. The basic structure of zeolites is biologically neutral.
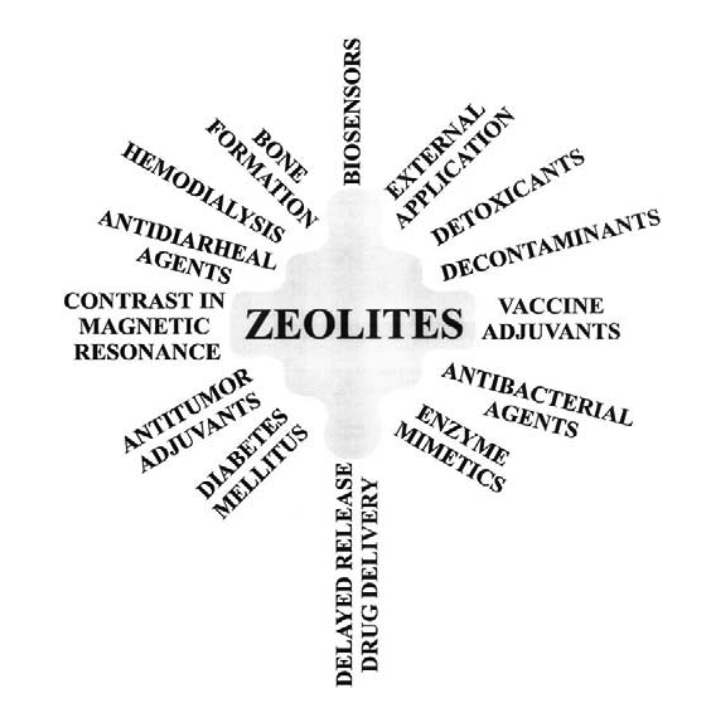
The ion-exchange process is reversible, allowing for adsorption of ions and mole- cules, making zeolites useful as filters for dust, toxin removal, and as chemical sieves. Zeolites can have water as part of their structure; after the water has been driven off by heating, the basic framework structure is left intact. Subsequently, other solutions can be put through the structure, and thus the zeolite acts as a delivery system for the new fluid. This process has been exploited and applied in medicine, farm animal feed, and other types of research. Nowadays zeolites compete with cation-exchange resins in water processing and in purification of wastewater and sewage. Zeolites have high cation exchange selectivities, good resistance to temperature and ionizing radiations, and excellent com- patibility with the environment. Therefore, zeolites are widely used in modern technology as selective adsorbents, molecular sieves, and particularly as catalysts. It is obvious that the ion sieving and other remarkable properties of zeolites will be utilized in the near future for the environmental and health care industries. The reasons for this are as follows: (a) zeolites have known biological properties alone with long-term chemical and biological tability (4); (b) they reversibly bind small molecules such as oxygen and nitric oxide (5,6); (c) they possess size and shape selectivities; (d) they offer the possibility of metalloenzyme mimicry; and (e) they have immunomodulatory activity (Fig. 1).
Researchers are even exploring the possibility that zeolites and feldspars played a major role in the beginning of ‘‘life,’’ i.e., life may have begun by catalytic assembly on a mineral surface. Catalysis and mineral surfaces might have generated replicating bio- polymers from simple chemicals supplied by meteorites, volcanic gases, and photo- chemical reactions (7). How could the first replicating and energy-supplying molecules have been assembled from simpler materials that were available on the early protoconti- nents? Concepts of catalysis that use organic compounds dispersed in aqueous ‘‘soups’’ require a mechanism for catalytic substrate. After catalysis, biochemically significant poly- mers such as polypeptides and RNA might have been protected from photochemical destruction by solar radiation. Assuming temperatures were not too high, energy-con- suming replication/mutation of polymers could have led to the first primitive organisms.
A new concept is that certain materials have internal surfaces that are both organophilic and catalytic, allowing efficient capture of organic species for catalytic assembly into polymers in a protective environment. Indeed, caution is warranted in proposing that life began merely as a trivial scientific event on a mineral catalyst (7). Nucleic acids can be adsorbed onto and preserved by clays, which are layered alumi- nosilicates. It is known that environmental DNA can be stabilized by adsorption onto sand and clay particles, thereby becoming 100- to 1000-fold more resistant to deoxy- ribonuclease (DNase). Such adsorbed DNA may retain its transforming ability for weeks and even months (8).
Since many biochemical processes are closely related to some zeolite properties (ion- exchange, adsorption, and catalysis), we believe that natural and synthetic zeolites may lead to significant advances in biology, medicine, and in the pharmaceutical industry in the near future.
source: scribd.com
![Ζεόλιθος και εξοικονόμηση θρεπτικών συστατικών και νερού [Διατριβή]](https://zeolife.gr/wp-content/uploads/2018/10/zeolite-agriculture-nutrients-water-3-768x432.jpg)